Photo courtesy of Apple
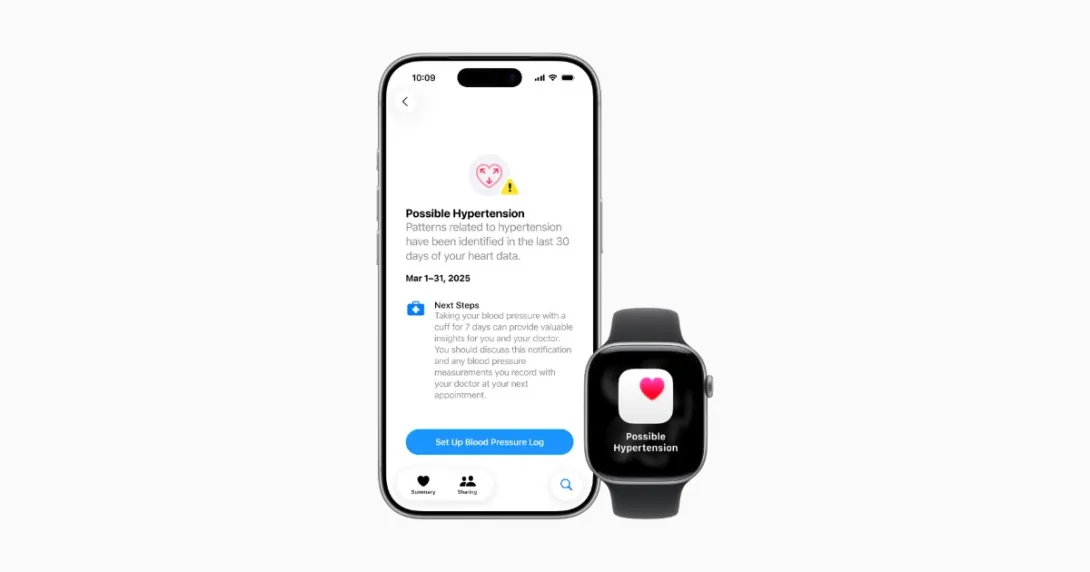
Apple has received clearance from the U.S. Food and Drug Administration (FDA) for its recently announced hypertension notification feature available on some of its smartwatches.
The company unveiled the feature, which detects signs of chronic high blood pressure, during its Sept. 9 event.
Apple said that with the feature, every 30 days an algorithm will analyze a user's data gathered from the optical heart sensor to evaluate how a user's blood vessels respond to heartbeats.
The watch will determine if signs consistent with hypertension exist and notify the user.
During its event last week, the tech giant said the hypertension notification feature was developed using machine learning and training data from various studies with more than 100,000 participants.
The performance of the feature was then validated in a clinical study with over 2,000 participants, Apple said.
The company stated during its event that the feature will not detect all instances of hypertension, but it expects to notify over one million people who may have undiagnosed hypertension of their potential disease within the first year.
The hypertension notification feature will be available on Apple Watch Series 9, Series 10, Series 11 and the tech giant's Watch Ultra 2 and Ultra 3 models with watch OS 26 in more than 150 countries, including the U.S. and European Union, before the end of September.
THE LARGER TREND
Apple has received numerous FDA clearances for its health features.
In June, the tech giant received 510(k) clearance for its Digital Prism Correction Feature (DPCF), software intended to provide digital image adjustments in the Apple Vision Pro in consonance with a user's prism eyeglass prescription.
Prism in eyeglass prescription refers to specialized lenses that bend light in a specific direction to correct certain vision problems. The lenses can help align the eyes to reduce double vision or eye strain.
Apple's DPCF "fulfills the prism part of the prescription while using Apple Vision Pro" and "supports prism prescriptions up to 7.75 Prism Diopters in the horizontal and/or vertical dimension."
Last year, Apple received FDA clearance for its sleep apnea detection feature that analyzes data from the Apple Watch sensor to detect signs of breathing disturbances suggestive of moderate to severe sleep apnea in users 18 and older.
The company also received FDA clearance for its over-the-counter hearing aid software in its AirPods Pro headphones, which include clinical-grade over-the-counter hearing aids that use machine learning to adjust noises to users' hearing needs automatically.
Wearers take a five-minute test using their iPhone and AirPods Pro to analyze their hearing health and tap their iPhone screen when they hear a series of tones at different volumes and frequencies.
Based on the results of that test, AirPods Pro automatically adjusts to a user's hearing needs, serving as a clinical-grade hearing aid.
In 2023, the company received clearance for its Irregular Rhythm Notification Feature, another Apple Watch feature that analyzes a user's pulse rate data to identify irregular heart rate rhythms suggestive of atrial fibrillation (AFib). The feature is intended for individuals 22 years and older, and is only usable when the user is still.