Public Policy
The startup also announced a new product called Clear Contracts that aims to improve the direct contracting process.
The digital mental health unicorn confirmed it received a grand jury subpoena from the U.S. Attorney's Office for the Eastern District of New York on the evening of May 4.
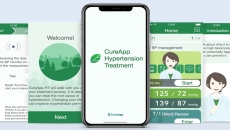
The clearance represents what could be the world's first regulatory approval of a DTx app for hypertension treatment.
Also: Pear Therapeutics receives FDA SteP designation for its product candidate focused on acute and chronic pain, and Carbon Health launches its diabetes management program.
The company said it's currently focused on managing end-stage kidney disease, but it's investigating using the system for other clinical areas.
This marks Aidoc's second FDA 510(k) so far this year.
The software automatically flags cases of pneumothorax, or a collapsed lung, on X-rays for more efficient triage.
A Nature article outlines the digital health approaches of nine countries around the world.
The Neural Sleeve uses electrical stimulation to help people with neurological conditions walk more easily.
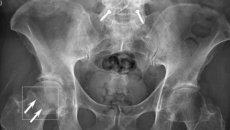
The BoneView software detects potential fractures in X-rays and submits them to radiologists for validation.