FDA
Data from the clinical trial might give patients a new option for treating migraines.
Ben Wolf, partner in Alston & Bird's Health Care Group, told MobiHealthNews how FDA staffing cuts could slow device approvals and what companies can do to stay ahead.
The judge ruled that the FDA overstepped its authority by regulating LDTs as medical devices, overturning the rule and remanding it to the HHS Secretary for review.
The firings included 20 individuals in the FDA's Office of Neurological and Physical Medicine Devices.
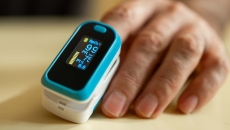
The new draft guidance aims to help improve the accuracy of pulse oximeters in patients across the range of skin pigmentations.
The Hyodol doll, widely marketed as a companion to solo-living seniors, has been registered with the US FDA.
The guidance recommends a PCCP describe the planned AI-DSF modifications, the associated methodology to develop, validate and implement them, and assess the impact of the changes.
The Agency says AI can transform multiple aspects of healthcare, but it is difficult to fully oversee and regulate the technology without the assistance of all sectors involved.
The Agency has qualified Apple's atrial fibrillation history feature as a medical device development tool, allowing it to be used in clinical trials.
The Agency alleges the company's CT and ultrasonography systems are noncompliant with good manufacturing requirements.